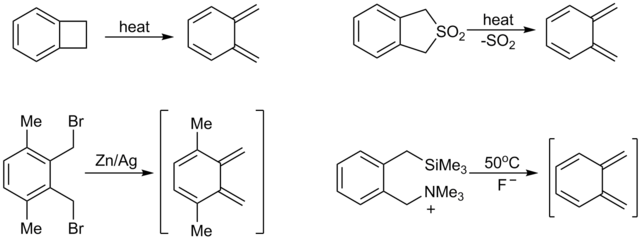
Xylylene
[8] For example, reaction of tetrabromo-o-xylene (C6H4(CHBr2)2) with sodium iodide affords α,α'-dibromo-o-xylylene, which can be trapped to give naphthylene derivatives.
In the absence of trapping agents, the xylylene relaxes to α,α'-dibromobenzocyclobutane:[9] Cycloadditions of these o-xylylenes provides a pathway to acenes.
For example, reaction of α,α'-dibromo-o-xylene with iron carbonyls affords low yields of the xylylene complex Fe(CO)3[η4-C6H4(CH2)2].
This and other syntheses of o-xylylenes, and their subsequent dimerization by [4+4] cycloaddition to form cycloctyl structures, were used repeatedly in the synthesis of superphane.
[12] Despite the observed chemistry of para-xylylene (i.e. its rapid polymerization to poly-p-xylylene), which suggests the compound exists as a diradical, physical evidence unanimously concludes that the lowest electronic state of p-xylylene is a closed shell singlet.