Zincke aldehyde
[1][2][3] The use of cyanogen bromide for pyridine activation was independently reported by W. König:[4] The synthesis and utility of Zincke aldehydes has been reviewed.
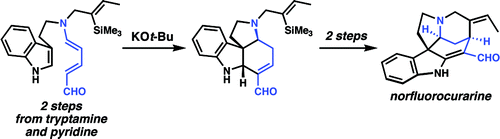
[5][6][7] A variation of the Zincke reaction has been applied in the synthesis of novel indoles:[8] with cyanogen bromide mediated pyridine activation (König method).
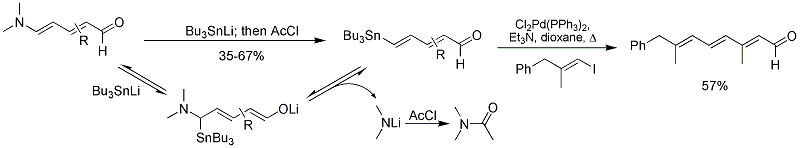
[4] More recently, an interesting rearrangement of Zincke aldehydes to Z-unsaturated amides was discovered serendipitously while trying to do an intramolecular Diels-Alder reaction.
[10] Mechanistic details were also discussed, however further investigations in collaboration with the Houk group revealed an unusual and unexpected mechanism based on computational studies.
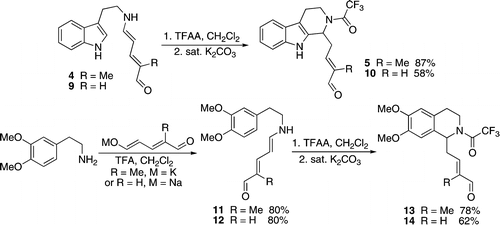
[15] This reaction provided the tetrahydro-β-carboline or tetrahydroisoquinoline core present in many alkaloid natural products, and was applied to the construction of a known intermediate in a previous total synthesis.