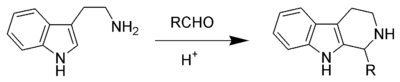
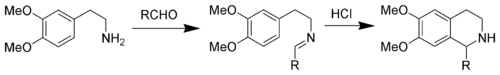
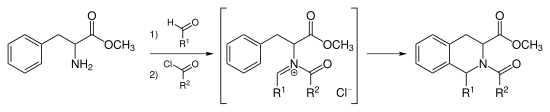
Pictet–Spengler reaction
The driving force for this reaction is the electrophilicity of the iminium ion generated from the condensation of the aldehyde and amine under acid conditions.
This explains the need for an acid catalyst in most cases, as the imine is not electrophilic enough for ring closure but the iminium ion is capable of undergoing the reaction.
It has remained an important reaction in the fields of alkaloid and organic synthesis since its inception, where it has been employed in the development of many beta-carbolines.
Nucleophilic aromatic rings such as indole or pyrrole give products in high yields and mild conditions, while less nucleophilic aromatic rings such as a phenyl group give poorer yields or require higher temperatures and strong acid.
[6] The reaction mechanism occurs by initial formation of an iminium ion (2) followed by electrophilic addition at the 3-position, in accordance with the expected nucleophilicity of indoles, to give the spirocycle 3.
The N-acyliminium ion is a very powerful electrophile and most aromatic ring systems will cyclize under mild conditions with good yields.