Zirconocene
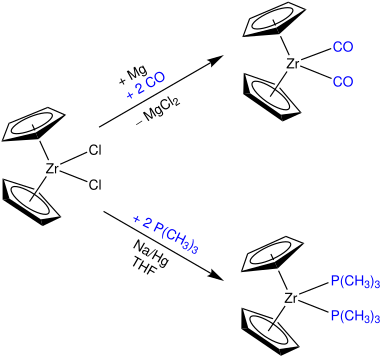
A crucial question in research is what kind of ligands can be used to stabilize the Cp2ZrII metallocene fragment to make it available for further reactions in organic synthesis.
[1] In contrast to sandwich compounds that have parallel cyclopentadienyl rings bound on opposite sides of the metal atom, such as ferrocene, zirconocene and other group 4 metallocenes are bent.
This reagent is stable at room temperature, can be stored under an inert atmosphere and allows a more precise control over the stoichiometry of reactions as it can be formed quantitatively.
These reactions have been observed upon addition of carbon monoxide, ketones, nitriles, alkynes and other substances and led to five-, seven- and nine-membered metallacycles.
[8] Moreover, with zirconocene complexes, the synthesis of so far unknown heterometallacycles and synthetically challenging organic structures can be realized by novel C-C coupling of nitriles.