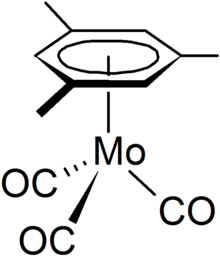(Mesitylene)molybdenum tricarbonyl
(Mesitylene)molybdenum tricarbonyl arises from the reaction of molybdenum hexacarbonyl with hot mesitylene:[1] It can also be synthesized, with good yields by displacement of pyridine ligands of the trispyridine complex Mo(CO)3(pyridine)3 in the presence of Lewis acids.
This reaction proceeds at lower temperatures of the compound than the direct method The mesitylene group is bonded to the molybdenum centre through delocalized π - electron ring.
[2][3] The arene can be displaced by the trimethylphosphite via a SN2 type mechanism to give the fac-tricarbonyltris(trimethyl phosphite)molybdenum.
The result of the charge transfer facilitates ring slippage and the mesitylene group changes from η6 to η2 this allows the phenylacetylene monomer units to bind to the metal centre.
Recently, it has been reported that tricarbonyl(mesitylene)molybdenum can act as a catalyst for the epoxidation of alkenes.