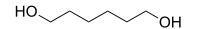
1,6-Hexanediol
[3] W. H. Perkin Jr. and his graduate student Edward Haworth first prepared the compound in 1894 during their research on cyclohexane.
They boiled 1,6-dibromohexane (which they were also the first to synthesize) with dilute potassium carbonate solution and named the product hexamethylene glycol.
[3][5] Laboratory preparation could be achieved by reduction of adipates with lithium aluminium hydride, although this method is impractical on a commercial scale.
As 1,6-hexanediol contains hydroxyl groups, it undergoes the typical chemical reactions of alcohols such as dehydration, substitution, and esterification.
1,6-Hexanediol has been reported to interfere with weak hydrophobic protein-protein or protein-RNA interactions that comprise liquid condensates.