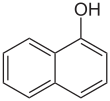
1-Naphthol
Some reactions of 1-naphthol are explicable with reference to its tautomerism, which produces a small amount of the keto tautomer.
[citation needed] One consequence of this tautomerism is the Bucherer reaction, the ammonolysis of 1-naphthol to give 1-aminonaphthalene.
This regioselective reaction is exploited in the preparation of diazo dyes, which are form using diazonium salts.
[4][5] Partial reduction of 1-naphthol gives the tetrahydro derivative, leaving intact the phenol ring.
[13] 1-Naphthol is used in each of the following chemical tests, which predate the use of spectroscopic and chromatographic methods: 1-Naphthol has been described as "moderately toxic".