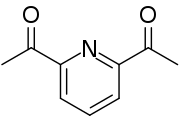
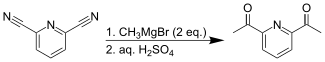2,6-Diacetylpyridine
[1][2] The synthesis of 2,6-diacetylpyridine begins with oxidation of the methyl groups in 2,6-lutidine to form dipicolinic acid.
[3] The diketone can be formed from the diester of picolinic acid groups through a Claisen condensation.
[2] Diacetylpyridine is a popular starting material for ligands in coordination chemistry, often via template reactions.
The diiminopyridine (DIP) class of ligands can be formed from diacetylpyridine through Schiff base condensation with substituted anilines.
Diiminopyridine ligands have been the focus of much interest due to their ability to traverse a wide range of oxidation states.