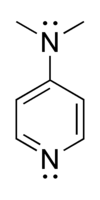
4-Dimethylaminopyridine
Chiral DMAP analogues are used in kinetic resolution experiments of mainly secondary alcohols and Evans auxiliary type amides.
This cation then reacts with dimethylamine:[7] In the case of esterification with acetic anhydrides the currently accepted mechanism involves three steps.
Here the acetate acts as a base to remove the proton from the alcohol as it nucleophilically adds to the activated acylpyridinium.
The described bond formation and breaking process runs synchronous concerted without the appearance of a tetrahedral intermediate.
In the last step of the catalytic cycle the auxiliary base (usually triethylamine or pyridine) deprotonates the protonated DMAP, reforming the catalyst.