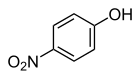
4-Nitrophenol
The beta-form is yellow pillars, stable at room temperature, and gradually turns red upon irradiation of sunlight.
The yellow color of the 4-nitrophenolate form (or 4-nitrophenoxide) is due to a maximum of absorbance at 405 nm (ε = 18.3 to 18.4 mM−1 cm−1 in strong alkali).
Accurate measurement of enzyme activity requires that the 4-nitrophenol product is fully deprotonated, existing as 4-nitrophenolate, given the weak absorbance of 4-nitrophenol at 405 nm.
Since the length of conjugated systems affects the color of organic compounds, this ionization change causes the 4-nitrophenol to turn yellow when fully deprotonated and existing as 4-nitrophenolate.
[8] A common mistake in measuring enzyme activity using these substrates is to perform the assays at neutral or acidic pH without considering that only part of the chromophoric product is ionized.
The problem can be overcome by stopping the reaction with sodium hydroxide (NaOH) or other strong base, which converts all product into 4-nitrophenoxide; final pH must be > ca.
It has a delayed interaction with blood and forms methaemoglobin which is responsible for methemoglobinemia, potentially causing cyanosis, confusion, and unconsciousness.