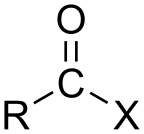
Acyl halide
[2] In organic chemistry, the term typically refers to acyl halides of carboxylic acids (−C(=O)OH), which contain a −C(=O)X functional group consisting of a carbonyl group (C=O) singly bonded to a halogen atom.
[9] For example, chloroformylation, a specific type of Friedel-Crafts acylation which uses formaldehyde as a reagent[citation needed], or by the direct chlorination of benzaldehyde derivatives.
[11] Aromatic (as well as aliphatic) acyl fluorides are conveniently prepared directly from carboxylic acids, using stable, inexpensive commodity chemicals: PPh3, NBS and Et3N-3HF in a bench-top protocol.
An important use of adipoyl chloride is polymerization with an organic di-amino compound to form a polyamide called nylon or polymerization with certain other organic compounds to form polyesters.
In general, acyl halides (even non-volatile compounds such as tosyl chloride) are irritants to the eyes, skin and mucous membranes.