Actinin
Simultaneously, the other duplicated gene acquired extra repeats through a series of unequal crossing-over events.
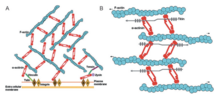
This made the spectrin subunit ancestor which is an antiparallel homodimer that can crosslink actin filaments.
[3] Alpha-actinin 1 (ACTN1) was discovered forty years ago due to it being present in the striated muscle contractile apparatus in large amounts.
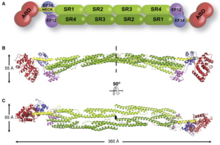
[1] It consists of an actin binding domain (ABD) connected to four spectrin repeats forming the central rod through a flexible neck region.
[8][1] This forms the binding site at each end of the protein which results in a rod-shaped molecule and with bundles of actin filaments.
[8] Alpha-actinin 3 (ACTN3) is deficient in around sixteen percent of humans and it plays a significant role in muscle metabolism.
[12] Among the four mammalian alpha-actinins, ACTN3 stands out as the most highly specialized, primarily expressed in fast glycolytic fibers within skeletal muscle.
[11] In humans that have ACTN3, scientists have seen better results in sprinting and power performance in athletes and the general population.
[10] Even though this has been found, recent positive selection appears to have influenced the null genotype XX, possibly owing to its emerging role in regulating muscle metabolism, as suggested by the available evidence.
[10] The lack of ACTN3 results in a more oxidative pathways of energy being used as glycogen phosphorylase activity is reduced.
ACTN4 guides the connection between the actin cytoskeleton within the cell and the integrins that directly interact with the stromal ECM.
[9] This process is crucial for the formation and continuation of breast, colorectal, ovarian, and pancreatic cancer.
Amoeboid type cells lack stress fibers and use high myosin II mediated contraction which allows for it to invade the blebbing mechanism.
[9] This process is what scientists are looking at further as this could clarify the significant rise in invasion and metastasis observed as they navigate through the dense stroma of tumors.