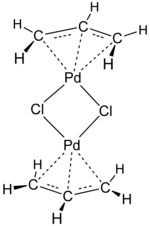
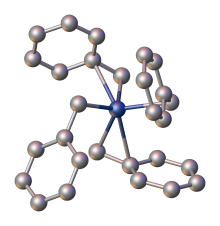
Transition-metal allyl complex
The η3-allyl group is classified as an LX-type ligand in the Green LXZ ligand classification scheme, serving as a 3e– donor using neutral electron counting and 4e– donor using ionic electron counting.
[1] 1,3-Dienes such as butadiene and isoprene dimerize in the coordination spheres of some metals, giving chelating bis(allyl) complexes.
Chelating bis(allyl) complexes are intermediates in the metal-catalyzed dimerization of butadiene to give vinylcyclohexene and cycloocta-1,5-diene.
In such circumstances, heating or irradiation can dislocate another ligand to free up space for the alkene-metal bond.
[3] However, the carbanion salt precursors require careful synthesis, as allyl halides readily undergo Wurtz coupling.
[14] X-ray crystallography demonstrate that the benzyl ligands in tetrabenzylzirconium are highly flexible.
[13] Allyl complexes are often discussed in academic research,[15][16][17][18] but few have commercial applications.