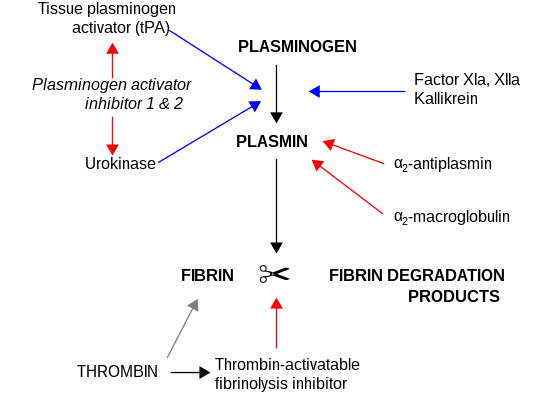
alpha-2-Macroglobulin
The concentration of α2-macroglobulin rises 10-fold or more in the nephrotic syndrome when other lower molecular weight proteins are lost in the urine.
The net result is that α2-macroglobulin reaches serum levels equal to or greater than those of albumin in the nephrotic syndrome, which has the effect of maintaining oncotic pressure.
[5][6] In addition to tetrameric forms of α2-macroglobulin, dimeric, and more recently monomeric αM protease inhibitors have been identified.
These protease inhibitors share several defining properties, which include (1) the ability to inhibit proteases from all catalytic classes, (2) the presence of a 'bait region' (also known as a sequence of amino acids in an α2-macroglobulin molecule, or a homologous protein, that contains scissile peptide bonds for those proteinases that it inhibits) and a thiol ester, (3) a similar protease inhibitory mechanism and (4) the inactivation of the inhibitory capacity by reaction of the thiol ester with small primary amines.
In the resulting αM-protease complex, the active site of the protease is sterically shielded, thus substantially decreasing access to protein substrates.
Two additional events occur as a consequence of bait region cleavage, namely (1) the h-cysteinyl-g-glutamyl thiol ester becomes highly reactive and (2) a major conformational change exposes a conserved COOH-terminal receptor binding domain [13] (RBD).
RBD exposure allows the αM protease complex to bind to clearance receptors and be removed from circulation.