Amine
In chemistry, amines (/əˈmiːn, ˈæmiːn/,[1][2] UK also /ˈeɪmiːn/[3]) are compounds and functional groups that contain a basic nitrogen atom with a lone pair.
Formally, amines are derivatives of ammonia (NH3(in which the bond angle between the nitrogen and hydrogen is 107°), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group[4] (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines).
Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure R−C(=O)−NR′R″, are called amides and have different chemical properties from amines.
An organic compound with multiple amino groups is called a diamine, triamine, tetraamine and so forth.
The nitrogen atom features a lone electron pair that can bind H+ to form an ammonium ion R3NH+.
Small aliphatic amines display significant solubility in many solvents, whereas those with large substituents are lipophilic.
Aromatic amines, such as aniline, have their lone pair electrons conjugated into the benzene ring, thus their tendency to engage in hydrogen bonding is diminished.
Typically the presence of an amine functional group is deduced by a combination of techniques, including mass spectrometry as well as NMR and IR spectroscopies.
[6] In their IR spectra, primary and secondary amines exhibit distinctive N-H stretching bands near 3300 cm−1.
For the case of propyl amine, the H-N-H scissor mode appears near 1600 cm−1, the C-N stretch near 1000 cm−1, and the R2N-H bend near 810 cm−1.
Amines of the type NHRR' and NRR′R″ are chiral: the nitrogen center bears four substituents counting the lone pair.
Because of the low barrier to inversion, amines of the type NHRR' cannot be obtained in optical purity.
In aromatic amines ("anilines"), nitrogen is often nearly planar owing to conjugation of the lone pair with the aryl substituent.
For anilines, the lone pair of electrons on nitrogen delocalizes into the ring, resulting in decreased basicity.
Substituents on the aromatic ring, and their positions relative to the amino group, also affect basicity as seen in the table.
The intrinsic basicity of amines, i.e. the situation where solvation is unimportant, has been evaluated in the gas phase.
In the gas phase, amines exhibit the basicities predicted from the electron-releasing effects of the organic substituents.
Similarly aniline is more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.
For this reason, the basicity of amines in these aprotic solvents is almost solely governed by the electronic effects.
Selectivity is also assured in the Gabriel synthesis, which involves organohalide reacting with potassium phthalimide.
Disubstituted alkenes react with HCN in the presence of strong acids to give formamides, which can be decarbonylated.
In the case of nitriles, reactions are sensitive to acidic or alkaline conditions, which can cause hydrolysis of the −CN group.
[16] Most primary amines are good ligands for metal ions to give coordination complexes.
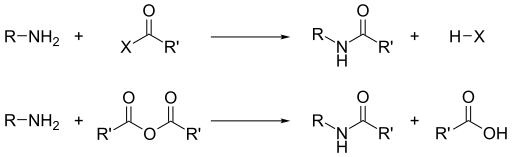
Acyl chlorides and acid anhydrides react with primary and secondary amines to form amides (the "Schotten–Baumann reaction").
[17] Diazonium salts undergo a variety of useful transformations involving replacement of the N2 group with anions.
The breakdown of amino acids releases amines, famously in the case of decaying fish which smell of trimethylamine.
As azo-compounds are highly coloured, they are widely used in dyeing industries, such as: Most drugs and drug candidates contain amine functional groups:[24] Aqueous monoethanolamine (MEA), diglycolamine (DGA), diethanolamine (DEA), diisopropanolamine (DIPA) and methyldiethanolamine (MDEA) are widely used industrially for removing carbon dioxide (CO2) and hydrogen sulfide (H2S) from natural gas and refinery process streams.
[29] The reaction proceeds by the lone pair of electrons on the amine nitrogen attacking the outermost carbon on the oxirane ring of the epoxy resin.
[30] Molecules with tertiary amine functionality are often used to accelerate the epoxy-amine curing reaction and include substances such as 2,4,6-Tris(dimethylaminomethyl)phenol.
It has been stated that this is the most widely used room temperature accelerator for two-component epoxy resin systems.