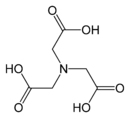
Aminopolycarboxylic acid
Aminopolycarboxylates that have lost acidic protons form strong complexes with metal ions.
This property makes aminopolycarboxylic acids useful complexone in a wide variety of chemical, medical, and environmental applications.
[2] Replacement of a hydrogen atom on the nitrogen of glycine by another acetate residue, –CH2COOH gives iminodiacetic acid, IDA, which is a tridentate ligand.
EDTA contains two IDA units with the nitrogen atoms linked by two methylene groups and is hexadentate.
The chelating properties of aminopolycarboxylates can be engineered by varying the groups linking the nitrogen atoms so as to increase selectivity for a particular metal ion.