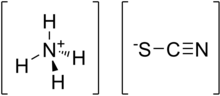Ammonium thiocyanate
Ammonium thiocyanate is used in the manufacture of herbicides, thiourea, and transparent artificial resins; in matches; as a stabilizing agent in photography; in various rustproofing compositions; as an adjuvant in textile dyeing and printing; as a tracer in oil fields; in the separation of hafnium from zirconium (important for the production of hafnium-free zircalloy for use in nuclear fuel cladding), and in titrimetric analyses.
Ammonium thiocyanate may also be used to separate quinidine, from liquors, after the isolation of quinine from the neutral, aqueous, sulphate solution.
The salt is added to the hot solution and the gummy solid that forms is strained off from the liquid.
Ammonium thiocyanate is made by the reaction of carbon disulfide with aqueous ammonia.
When heated at 200 °C, the dry powder decomposes to ammonia, hydrogen sulfide, and carbon disulfide, leaving a residue of guanidinium thiocyanate.