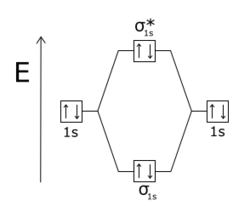
Antibonding molecular orbital
The density of the electrons in the orbital is concentrated outside the bonding region and acts to pull one nucleus away from the other and tends to cause mutual repulsion between the two atoms.
The Pauli exclusion principle prohibits any two electrons (e-) in a molecule from having the same set of quantum numbers.
A molecular orbital becomes antibonding when there is less electron density between the two nuclei than there would be if there were no bonding interaction at all.
In homonuclear diatomic molecules, σ* (sigma star) antibonding orbitals have no nodal planes passing through the two nuclei, like sigma bonds, and π* (pi star) orbitals have one nodal plane passing through the two nuclei, like pi bonds.
The Pauli exclusion principle dictates that no two electrons in an interacting system may have the same quantum state.
Roald Hoffmann and Kenichi Fukui shared the 1981 Nobel Prize in Chemistry for their work and further development of qualitative molecular orbital explanations for chemical reactions.