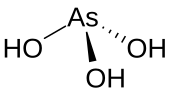
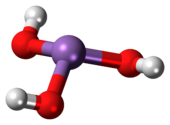
Arsenous acid
It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)3.
The differing behaviors of the As and P compounds reflect a trend whereby high oxidation states are more stable for lighter members of main group elements than their heavier congeners.
[4] Reactions attributed to aqueous arsenic trioxide are due to arsenous acid and its conjugate bases.
For example, it reacts with hydrochloric, hydrobromic, and hydroiodic acids to produce arsenic trichloride, tribromide, and triiodide.
The anhydride form of arsenous acid, arsenic trioxide, is used as a herbicide, pesticide, and rodenticide.