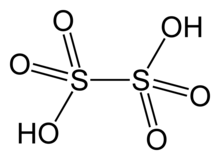
Dithionic acid
Dithionic acid, H2S2O6, is the inorganic compound with the formula H2S2O6.
It is the doubly protonated derivative of dithionate, a well-characterized dianion.
Dithionic acid is mainly observed and characterized as an aqueous solution.
[3] Dithionates can be made by oxidizing a sulfite (from the +4 to the +5 oxidation state), but on a larger scale they are made by oxidizing a cooled aqueous solution of sulfur dioxide with manganese dioxide: The manganese dithionate solution formed can then be converted to dithionate salts of other metals by metathesis reactions: Concentrated solutions of dithionic acid can subsequently be obtained treating a barium dithionate solution with sulfuric acid: