Sulfamic acid
[5] Furthermore, a neutron diffraction study located the hydrogen atoms, all three of which are 1.03 Å distant from the nitrogen.
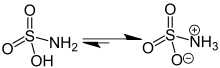
The crystalline solid is indefinitely stable under ordinary storage conditions, however, aqueous solutions of sulfamic acid slowly hydrolyse to ammonium bisulfate, according to the following reaction: Its behaviour resembles that of urea, (H2N)2CO.
Both feature amino groups linked to electron-withdrawing centres that can participate in delocalised bonding.
It is more expensive than other reagents for doing this, such as chlorosulfonic acid or oleum, but is also significantly milder and will not sulfonate aromatic rings.
An example of this reaction is the production 2-ethylhexyl sulfate, a wetting agent used in the mercerisation of cotton, by combining sulfamic acid with 2-ethylhexanol.
It is frequently used for removing rust and limescale, replacing the more volatile and irritating hydrochloric acid, which is cheaper.
It is often a component of household descalant, for example, Lime-A-Way Thick Gel contains up to 8% sulfamic acid and has pH 2.0–2.2,[12] or detergents used for removal of limescale.
If inadvertently mixed with hypochlorite based products such as bleach, it does not form chlorine gas, whereas the most common acids would; the reaction (neutralisation) with ammonia, produces a salt, as depicted in the section above.