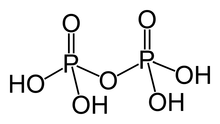
Pyrophosphoric acid
Colorless and odorless, it is soluble in water, diethyl ether, and ethyl alcohol.
[1] Anions, salts, and esters of pyrophosphoric acid are called pyrophosphates.
[3] Pyrophosphoric acid is a tetraprotic acid, with four distinct pKa's:[4] The pKa's occur in two distinct ranges because deprotonations occur on separate phosphate groups.
At physiological pH's, pyrophosphate exists as a mixture of doubly and singly protonated forms.
The percentage by weight of pyrophosphoric acid is around 40% and it is difficult to recrystallise from the melt.