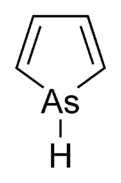
Arsole
Calculations suggest that whereas pyrrole (C4H4NH) molecule is planar, phosphole (C4H4PH) and heavier metalloles are not, and their pnictogen-bonded hydrogen atom extends out of plane.
However, the planarity is evaluated in calculation by the energy required to convert between the two configurations where the M-H bond is extending left or right from the molecular plane.
However, non-zero (small) value of this energy does not necessarily mean the molecule has low symmetry, because of the possibility of thermal or quantum tunneling between the two configurations.
[10] Aromaticity of arsole and its derivatives has been debated for years both from experimental and theoretical points of view.
[11] Substitution of all hydrogen atoms in arsole with phenyl groups yields yellow needles of crystalline pentaphenylarsole, which has a melting point of 215 °C.
Substituting in this reaction arsenic trichloride for phenylarsenous dichloride yields 1-chloro-2,3,4,5-tetraphenylarsole, which also forms yellow needles but with a lower melting point of 182–184 °C.