Bent's rule
Bent as follows:[2] Atomic s character concentrates in orbitals directed toward electropositive substituents.Valence bond theory gives a good approximation of molecular structure.
By removing the assumption that all hybrid orbitals are equivalent, Bent's rule leads to improved predictions of molecular geometry and bond strengths.
Bent's rule represents a modification of VSEPR theory for molecules of lower than ideal symmetry.
In the early 1930s, shortly after much of the initial development of quantum mechanics, those theories began to be applied towards molecular structure by Pauling,[7] Slater,[8] Coulson,[9] and others.
On the one hand, a lone pair (an occupied nonbonding orbital) can be thought of as the limiting case of an electropositive substituent, with electron density completely polarized towards the central atom.
Bent's rule predicts that, in order to stabilize the unshared, closely held nonbonding electrons, lone pair orbitals should take on high s character.
On the other hand, an unoccupied (empty) nonbonding orbital can be thought of as the limiting case of an electronegative substituent, with electron density completely polarized towards the ligand and away from the central atom.
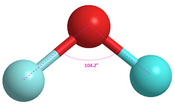
Experimentally, the first conclusion is in line with the reduced bond angles of molecules with lone pairs like water or ammonia compared to methane, while the second conclusion accords with the planar structure of molecules with unoccupied nonbonding orbitals, like monomeric borane and carbenium ions.
After determining how the hybridisation of the central atom should affect a particular property, the electronegativity of substituents can be examined to see if Bent's rule holds.
Valence shell electron pair repulsion (VSEPR) theory predicts molecule geometry.
[11][12] Bent's rule states "[A]tomic s character concentrates in orbitals directed toward electropositive substituents".
[5] VSEPR theory suggests a way to accurately predict molecule shape using simple rules.
[5] Furthermore, it has been shown that Bent's rule corroborates quantum mechanical computations when describing molecule geometry.
According to VSEPR theory, diethyl ether, methanol, water and oxygen difluoride should all have a bond angle of 109.5o.
Dimethyl ether, methanol, water and oxygen difluoride follow this trend as expected (as is shown in the table above).
Finally, when both hydrogen substituents are replaced with fluorine in oxygen difluoride, there is another decrease in the bond angle.
Against the expectations of VSEPR theory but consistent with Bent's rule, the bond angles of ammonia (NH3) and nitrogen trifluoride (NF3) are 107° and 102°, respectively.
Unlike VSEPR theory, whose theoretical foundations now appear shaky, Bent's rule is still considered to be an important principle in modern treatments of bonding.
By adding electronegative substituents and changing the hybridisation of the central atoms, bond lengths can be manipulated.
A prediction based on sterics alone would lead to the opposite trend, as the large chlorine substituents would be more favorable far apart.
As the steric explanation contradicts the experimental result, Bent's rule is likely playing a primary role in structure determination.
Perhaps the most direct measurement of s character in a bonding orbital between hydrogen and carbon is via the 1H−13C coupling constants determined from NMR spectra.
[25] The inductive effect is the transmission of charge through covalent bonds and Bent's rule provides a mechanism for such results via differences in hybridisation.
Orbital hybridisation allowed valence bond theory to successfully explain the geometry and properties of a vast number of molecules.
Henry Bent originally proposed his rule in 1960 on empirical grounds, but a few years later it was supported by molecular orbital calculations by Russell Drago.
[11][5] Bent's rule provides a reliable and robust framework for predicting the bond angles of molecules.
Bent's rule accuracy and precision in predicting the geometry of real-world molecules continues to demonstrate its credibility.
Bent's rule can be used to predict which products are favored in an organic synthesis depending on the starting materials.
al. considered how the substituents affected the silabenzenes' equilibrium and found that Bent's rule played a significant role in the results.
Knowing how molecular geometry accurately due to Bent's rule allows synthetic chemists to predict relative product stability.