Boroxine
Boroxine (B3H3O3) is a 6-membered heterocyclic compound composed of alternating oxygen and singly-hydrogenated boron atoms.
The interaction between the vacant p-orbitals in the boron atoms and the π-electrons in the aromatic, phenyl-substituents cause a different crystal structure.
This arrangement allows the phenyl-substituents to donate π-electron density to the vacant boron p-orbitals.
[8] As discovered in the 1930s, substituted boroxines (cyclo-(RBO)3, R = alkyl or aryl) are generally produced from their corresponding boronic acids by dehydration.
The trimethylbroxine appears to shift the equilibrium of the reaction as a dehydrating agent and facilitates the growth of COF material.
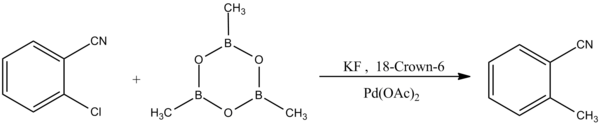
[9] Trimethylboroxine can be synthesized by reacting carbon monoxide with diborane (B2H6) and lithium borohydride (LiBH4) as a catalyst (or reaction of borane–tetrahydrofuran or borane–(dimethyl sulfide) in the presence of sodium borohydride):[5][10] Trimethylboroxine is used in the methylation of various aryl halides through palladium-catalyzed Suzuki-Miyaura coupling reactions:[11] Another form of the Suzuki-Miyaura coupling reaction exhibits selectivity to aryl chlorides:[12]