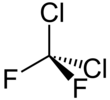
Dichlorodifluoromethane
It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride: This reaction can also produce trichlorofluoromethane (CCl3F), chlorotrifluoromethane (CClF3) and tetrafluoromethane (CF4).
[6] Charles F. Kettering, vice president of General Motors Research Corporation, was seeking a refrigerant replacement that would be colorless, odorless, tasteless, nontoxic, and nonflammable.
He assembled a team that included Thomas Midgley Jr., Albert Leon Henne, and Robert McNary.
From 1930 to 1935, they developed dichlorodifluoromethane (CCl2F2 or R12), trichlorofluoromethane (CCl3F or R11), chlorodifluoromethane (CHClF2 or R22), trichlorotrifluoroethane (CCl2FCClF2 or R113), and dichlorotetrafluoroethane (CClF2CClF2 or R114), through Kinetic Chemicals which was a joint venture between DuPont and General Motors.
[9] R-12 was used in most refrigeration and vehicle air conditioning applications prior to 1994 before being replaced by 1,1,1,2-tetrafluoroethane (R-134a), which has an insignificant ozone depletion potential.