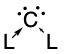Carbones
[1] These carbon-based compounds are of the formula CL2 where L is a strongly σ-donating ligand, typically a phosphine (carbodiphosphoranes) or a N-heterocyclic carbene/NHC (carbodicarbenes), that stabilises the central carbon atom through donor-acceptor bonds.
[2] Carbones possess high proton affinities[3][4] and are strong nucleophiles which allows them to function as ligands in a variety of main group and transition metal complexes.
[4] This is suggestive of a donor-acceptor interaction between the N-heterocyclic carbene ligands and a formally carbon (0) atom with two free lone pairs.
[2] The ease of bending and relatively large contribution of carbon in the two highest-occupied molecular orbitals imply a certain degree of carbone-like character in spite of the linear geometry.
Alternatively, a halide-substituted phosphonium salt can undergo an elimination reaction in the presence of a strong base to form a carbodiphosphorane.
[21] The first carbodicarbene synthesis was achieved much later than the first carbodiphosphorane synthesis, in 2008 by Dyker et al.[14] The first step was the methylation of bis(N-methylbenzimidazol-2-yl)methane using methyl triflate and the second step was the deprotonation of the carbon (II) species using potassium bis(trimethylsilyl)amide (KHMDS) to yield the desired N-heterocyclic-carbene-substituted carbone.
[22] Additionally, a method of facile synthesis of asymmetric carbodicarbenes was developed by Chen et al. in 2015 by using a simple nucleophilic substitution reaction.
[5] Experimentally, a variety of metal-carbodiphosphorane complexes have been synthesised and characterised, including with metals such as tungsten,[20] nickel,[25] copper,[26] silver,[26] and gold.
[22] In the former experiment, when a rhodium carbonyl complex was coordinated to a carbodicarbene, the carbon-oxygen stretching frequency was observed at 2014 cm−1 which is significantly lower than the same carbon-oxygen stretching frequency when rhodium is coordinated to a N-heterocyclic carbene (between 2058 cm−1 and 2036 cm−1) which is indicative of a strong π-donating effect from the second carbon lone-pair of the carbone.
The strong σ- and π-donating properties of carbones have made them optimal tools for stabilising reactive main-group-based species.