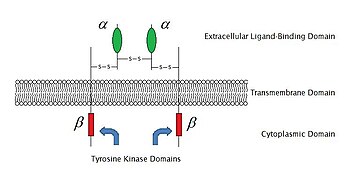
Cell surface receptor
In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.
If the polypeptide chain crosses the bilayer several times, the external domain comprises loops entwined through the membrane.
For example, a neurotransmitter, hormone, or atomic ions may each bind to the extracellular domain as a ligand coupled to receptor.
In other receptors, the transmembrane domains undergo a conformational change upon binding, which affects intracellular conditions.
In some receptors, such as members of the 7TM superfamily, the transmembrane domain includes a ligand binding pocket.
The intracellular (or cytoplasmic) domain of the receptor interacts with the interior of the cell or organelle, relaying the signal.
During the signal transduction event in a neuron, the neurotransmitter binds to the receptor and alters the conformation of the protein.
[15] The ligands which bind and activate these receptors include: photosensitive compounds, odors, pheromones, hormones, and neurotransmitters.
If the membrane receptors are denatured or deficient, the signal transduction can be hindered and cause diseases.
[18] Also, the cortical NMDA receptor influences membrane fluidity, and is altered in Alzheimer's disease.
In the case of poliovirus, it is known in vitro that interactions with receptors cause conformational rearrangements which release a virion protein called VP4.The N terminus of VP4 is myristylated and thus hydrophobic【myristic acid=CH3(CH2)12COOH】.
It is proposed that the conformational changes induced by receptor binding result in the attachment of myristic acid on VP4 and the formation of a channel for RNA.
In each case, a large number of potential ligand molecules are screened to find those fitting the binding pocket of the receptor.
The key advantage of searching a database is that it saves time and power to obtain new effective compounds.
In this case, ligand molecules are engineered within the constraints of a binding pocket by assembling small pieces in a stepwise manner.
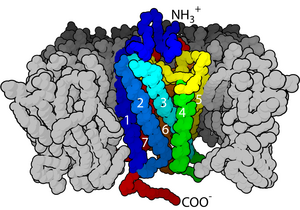

P = plasma membrane
I = intracellular space