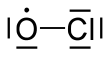Chlorine monoxide
It plays an important role in the process of ozone depletion.
The nonreactive nature of CFCs allows them to pass into the stratosphere, where they undergo photo-dissociation to form Cl radicals.
These then readily form chlorine monoxide, and this cycle can continue until two radicals react to form dichlorine monoxide, terminating the radical reaction.
Because the concentration of CFCs in atmosphere is very low, the probability of a terminating reaction is exceedingly low, meaning each radical can decompose many thousands of molecules of ozone.
This causes many chlorine radicals to be produced and hence a significant amount of ozone molecules are decomposed before the chlorine radicals are able to react with chlorine monoxide to form dichlorine monoxide.