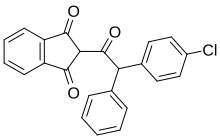
Chlorophacinone
Chlorophacinone belongs to the first-generation anticoagulant rodenticide group, first being developed during the 1940s to 1960s to control rodents in terrestrial environments.
Henry's law constant of 5.12 x 10−7 atm-m3/mol suggests a low potential to volatilize from water or soil into the atmosphere.
No purification is needed to start the last step of the synthesis, which is a Friedel-Crafts reaction of the previously obtained compound 4 with 1,3- indanedione 5.
[4] Synthesis of clotting factor II, VII, IX and X involves the posttranslational carboxylation of glutamate to γ-carboxyglutamate by the enzyme γ-glutamyl carboxylase (GGCX).
The γ-carboxyglutamate residues promote the binding of clotting factors to phospholipids of the blood vessels, thereby accelerating coagulation.
[9][10] Resulting in under-carboxylation of clotting factors, meaning they are no longer capable of binding to the endothelial surface of blood vessels, and thus are biologically inactive.
Hydroxylation occurs on the phenyl and indandionyl rings,[13] these metabolites can then further undergo conjugation with glucuronic acid prior to entering the systemic circulation, with potential enterohepatic recirculation.
[16] Belonging to the group of first-generation anticoagulant rodenticides, chlorophacinone has similar symptoms on animals as the other chemicals in its category.
Specifically, after being ingested several times by the target animal (most often a rodent), it interferes with the clotting of the blood and leads to internal bleeding, eventually causing death within 5 to 7 days.
This effect is due to the rodenticide's inhibition of the vitamin K(1)-2,3 epoxide reductase (VKOR) enzyme which is responsible for the synthesis of vitamin K and therefore the clotting factors II, VII, IX and X, factors critical to blood clotting, lack of which eventually causes mass hemorrhage inside the animal.
[4] Accidental exposure incidents involving lambs have shown symptoms including epistaxis, respiratory distress, and facial and cervical swelling.
Post-mortem examination in two of the affected lambs have revealed that all organs had a pale appearance, notably the liver, and that the lungs were heavier than usual and were slightly brownish.
Birds are not as sensitive to chlorophacinone as mammals, but they may still experience sublethal effects from it, such as external bleeding, internal hematoma and increased blood coagulation time.
One was a moderate severity case, which involved an insulation worker being exposed to chlorophacinone dust by touching and/or inhaling it.
The worker experienced shakiness, fever, and vomiting, as well as respiratory, neurological, gastrointestinal, renal and cardiovascular symptoms.
The anticoagulant concentration is diluted ten-fold in secondary exposure, and even more when the predator also eats non-poisoned prey.