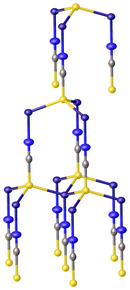
Copper(I) thiocyanate
The sulfur end of the SCN- ligand is triply bridging so that the coordination sphere for copper is CuS3N.
Copper(I) thiocyanate forms one double salt with the group 1 elements, CsCu(SCN)2.
As with CsCu (SNC)2, copper(I) thiocyanate separates out when these mixed salts are redissolved or their solutions diluted.
[10] Copper(I) thiocyanate is a hole conductor, a semiconductor with a wide band gap (3.6 eV, therefore transparent to visible and near infrared light).
[12] CuSCN with NiO act synergically as a smoke suppressant additive in polyvinyl chloride (PVC).