Copper(I) oxide
Alternatively, it may be prepared via the reduction of copper(II) acetate with hydrazine:[4] Aqueous cuprous chloride solutions react with base to give the same material.
These sugars reduce an alkaline solution of a copper(II) salt, giving a bright red precipitate of Cu2O.
It forms on silver-plated copper parts exposed to moisture when the silver layer is porous or damaged.
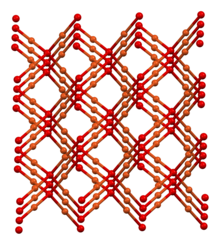
The structure thus resembles in some sense the main polymorphs of SiO2, but cuprous oxide's lattices interpenetrate.
Rectifier diodes based on this material have been used industrially as early as 1924, long before silicon became the standard.
Thus, light moves almost as slowly as sound in this medium, which results in high polariton densities.
Another unusual feature of the ground state excitons is that all primary scattering mechanisms are known quantitatively.
[11] In December 2021, Toshiba disclosed a transparent cuprous oxide (Cu2O) thin-film solar cell.