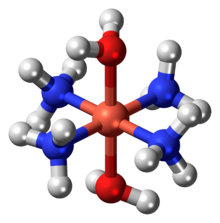
Schweizer's reagent
Evaporation of these solutions leaves light blue residue of copper hydroxide, reflecting the lability of the copper-ammonia bonding.
If conducted under a stream of ammonia, then deep blue needle-like crystals of the tetrammine form.
Cellulose, which is quite insoluble in water (hence its utility as clothing), dissolves in the presence of Schweizer's reagent.
The French chemist Louis-Henri Despeissis then proposed a procedure where cellulose is extruded into diluted sulphuric acid.
This leads to the complex no longer being stable enough to hold the cellulose in solution and it precipitates out forming strings.