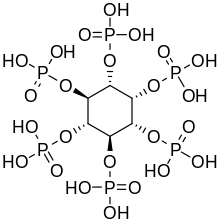
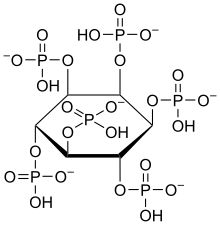
Phytic acid
The (myo) phytate anion is a colorless species that has significant nutritional role as the principal storage form of phosphorus in many plant tissues, especially bran and seeds.
Phytic acid and phytate have a strong binding affinity to the dietary minerals calcium, iron, and zinc, inhibiting their absorption in the small intestine.
[3] In most commercial agriculture, non-ruminant livestock, such as swine, fowl, and fish,[4] are fed mainly grains, such as maize, legumes, and soybeans.
[10] Although indigestible for many animals as they occur in seeds and grains, phytic acid and its metabolites have several important roles for the seedling plant.
Being not directly absorbed in the gut, phytic acid is not obtained from the animal diet, but must be synthesized inside the cell from phosphate and inositol (which in turn is produced from glucose, usually in the kidneys).
Proteolytic cleavage then unmasks an alternative binding site, where IP6 interaction promotes the assembly of the mature capsid lattice.
[16] IP6 has potential use in endodontics, adhesive, preventive, and regenerative dentistry, and in improving the characteristics and performance of dental materials.
[21] No detectable phytate (less than 0.02% of wet weight) was observed in vegetables such as scallion and cabbage leaves or in fruits such as apples, oranges, bananas, or pears.
[25] Phytic acid has a strong affinity to the dietary trace elements, calcium, iron, and zinc, inhibiting their absorption from the small intestine.