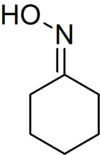Cyclohexanone oxime
This colorless solid is an important intermediate in the production of nylon 6, a widely used polymer.
This method is advantageous as cyclohexane is much cheaper than cyclohexanone.
The most famous and commercially important reaction of cyclohexanone oxime is Beckmann rearrangement yielding ε-caprolactam, which is used to produce Nylon 6: This reaction is catalyzed by sulfuric acid,[1] but industrial scale reactions use solid acids.
[2] Typical of oximes, the compound can be reduced by sodium amalgam to produce cyclohexylamine.
[3] It can also be hydrolyzed with acetic acid to give back cyclohexanone.