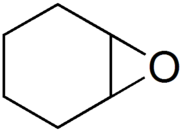Cyclohexene oxide
The epoxidation can take place either in a homogeneous reaction by peracids[2] or heterogeneous catalysis (e.g. silver and molecular oxygen).
A short overview and an investigation of the oxidation of cyclohexene by hydrogen peroxide is given in the literature.
[6] In recent times the catalytic oxidation of cyclohexene by (immobilized) metalloporphyrin complexes has been found to be an efficient way.
[7][8] In laboratory, cyclohexene oxide can also be prepared by reacting cyclohexene with magnesium monoperoxyphthalate (MMPP) in a mixture of isopropanol and water as solvent at room temperature.
[10] Cyclohexene oxide can be polymerized in solution, catalyzed by a solid acid catalyst.