Cyclooxygenase-2
It is involved in the conversion of arachidonic acid to prostaglandin H2, an important precursor of prostacyclin, which is expressed in inflammation.
[7] The two 15-HETE stereoisomers have intrinsic biological activities but, perhaps more importantly, can be further metabolized to a major class of agents, the lipoxins.
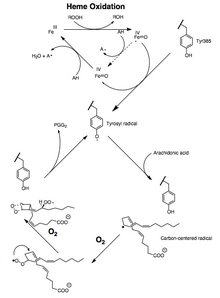
This is supported by the following evidence: 1) a significant kinetic isotope effect is observed for the abstraction of the 13-pro (S)-hydrogen; 2) carbon-centered radicals are trapped during catalysis;[16] 3) small amounts of oxidation products are formed due to the oxygen trapping of an allylic radical intermediate at positions 13 and 15.
PTGS1 (COX-1) and PTGS2 (COX-2) are bifunctional enzymes that carry out two consecutive chemical reactions in spatially distinct but mechanistically coupled active sites.
Both the cyclooxygenase and the peroxidase active sites are located in the catalytic domain, which accounts for approximately 80% of the protein.
Studies suggest that the concentration and composition of the free fatty acid pool in the environment in which PGHS-2 functions in cells, also referred to as the FA tone, is a key factor regulating the activity of PGHS-2 and its response to PTGS (COX) inhibitors.
Since PTGS2 (COX-2) is generally expressed only in cells where prostaglandins are upregulated (e.g., during inflammation), drug-candidates that selectively inhibit PTGS2 (COX-2) were suspected to show fewer side-effects[24] but proved to substantially increase risk for cardiovascular events such as heart attack and stroke.
Low-dose aspirin protects against heart attacks and strokes by blocking PTGS1 (COX-1) from forming a prostaglandin called thromboxane A2.
Prostacyclin relaxes or unsticks platelets, so selective COX-2 inhibitors (coxibs) increase risk of cardiovascular events due to clotting.
[31] Cyclooxygenases blocking by lornoxicam in acute stage of inflammation reduced the frequency of membrane formation by 43% in the dispase model of PVR and by 31% in the concanavalin one.
Lornoxicam not only normalized the expression of cyclooxygenases in both models of PVR, but also neutralized the changes of the retina and the choroid thickness caused by the injection of pro-inflammatory agents.
Increased expression of the PTGS2 gene in the fetal membranes is connected to the presence of inflammation, causing uterine prostaglandin gene expression and immunolocalization of prostaglandin pathway proteins in chorionic trophoblast cells and adjacent decidua, or choriodecidua.
[33] The mutant allele PTGS2 5939C carriers among the Han Chinese population have been shown to have a higher risk of gastric cancer.
[35] PTGS2 (COX-2) was discovered in 1991 by the Daniel Simmons laboratory[36][better source needed] at Brigham Young University.