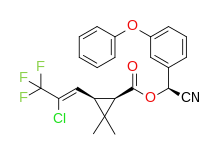
Cyhalothrin
Pyrethroids, such as cyhalothrin, are often preferred as an active ingredient in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins.
λ-and γ-cyhalothrin are now used to control insects and spider mites in crops including cotton, cereals, potatoes and vegetables.
By 1974, a team of Rothamsted Research scientists had discovered three pyrethroids (MoA 3a),[8] suitable for use in agriculture, namely permethrin, cypermethrin and deltamethrin.
Imperial Chemical Industries (ICI) obtained licenses to permethrin and cypermethrin but their agreement with the NRDC did not allow worldwide sales.
Also, it was clear to ICI's own researchers at Jealott's Hill that future competition in the marketplace might be difficult owing to the greater potency of deltamethrin compared to the other compounds.
For that reason, in the period 1974–1977, chemists there sought patentable analogues which might have advantages compared to the Rothamsted insecticides by having wider spectrum or greater cost-benefit.
The resulting material was found to be more potent than cypermethrin, to which it is most closely related, but also with good activity against the spider mite Tetranychus urticae, which added to its attractiveness as a potential new product.
[12] The process work made available a relatively large supply of the Z-cis acid and hence allowed two further commercially important steps to be taken.
After further research and field tests, ICI chose to focus on λ-cyhalothrin, the mixture containing the single most active isomer together with its mirror image.
This interior is shaped precisely to allow sodium ions to pass through the membrane, enter the axon, and propagate an action potential.
Since the active ingredient has very low solubility in water, formulations aid its use in water-based sprays by creating an emulsion when diluted.
Modern products use non-powdery formulations with reduced or no use of hazardous solvents: Examples include the capsule suspensions Warrior II[19] and Tandem, a mix with thiamethoxam,[20] both sold by Syngenta in the USA.
The purpose of the label is "to provide clear directions for effective product performance while minimizing risks to human health and the environment".
Once an active ingredient has achieved registration in major territories, suppliers often expand the market by seeking label approval[23] for additional crops and pests, after field trials have been carried out to confirm the efficacy of the product in the new situation.
[29][30] The Codex Alimentarius database maintained by the FAO lists the maximum residue limits for cyhalothrin isomers in various food products.
[31] While cyhalothrin is inherently highly toxic to many fish and aquatic invertebrate species, binding to soil and sediment reduces exposure and lessens the risk to fish: field studies found no significant adverse effects: according to the WHO expert committee, "The concentrations of cyhalothrin and lambda-cyhalothrin that are likely to arise in water from normal agricultural application will be low.
Since the compound is rapidly adsorbed and degraded under natural conditions, there will not be any practical problems concerning the accumulation of residues or the toxicity of cyhalothrin or lambda-cyhalothrin in aquatic species.
[37] In some cases, the risks of resistance developing can be reduced by using a mixture of two or more insecticides which each have activity on relevant pests but with unrelated mechanisms of action.