Cystathionine beta synthase
[7] The human enzyme cystathionine β-synthase is a tetramer and comprises 551 amino acids with a subunit molecular weight of 61 kDa.
The C-terminal domain of cystathionine β-synthase regulates its activity via both intrasteric and allosteric effects and is important for maintaining the tetrameric state of the protein.
[9] This inhibition is alleviated by binding of the allosteric effector, adoMet, or by deletion of the regulatory domain; however, the magnitude of the effects differ.
D. melanogaster and D. discoides have truncated N-terminal extensions and therefore prevent the conserved histidine and cysteine heme ligand residues.
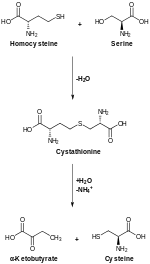
[12] CBS occupies a pivotal position in mammalian sulfur metabolism at the homocysteine junction where the decision to conserve methionine or to convert it to cysteine via the transsulfuration pathway, is made.
[9] In analogy with other β-replacement enzymes, the reaction catalyzed by CBS is predicted to involve a series of adoMet-bound intermediates.
[9] The final product, L-cystathionine can also form an aminoacrylate intermediate, indicating that the entire reaction of CBS is reversible.
[9] One of the alternate reactions involving CBS is the condensation of cysteine with homocysteine to form cystathionine and hydrogen sulfide (H2S).
In other words, AdoMet stimulates CBS activity by increasing the turnover rate rather than the binding of substrates to the enzyme.
[15] Human CBS performs a crucial step in the biosynthetic pathway of cysteine by providing a regulatory control point for AdoMet.
If the resting form of CBS in the cell has ferrous (Fe2+) heme, the potential exists for activating the enzyme under oxidizing conditions by conversion to the ferric (Fe3+) state.
Down syndrome is a medical condition characterized by an overexpression of cystathionine beta synthase (CBS) and a low level of homocysteine in the blood.
Pharmacologicals inhibitors of CBS have been patented by the Jerome Lejeune Foundation (November 2011) and trials (animals and humans are planned).
Genetic defects that affect the MTHFR, MTR, and MTRR/MS enzyme pathways can also contribute to high homocysteine levels.
Inborn errors in CBS result in hyperhomocysteinemia with complications in the cardiovascular system leading to early and aggressive arterial disease.
[16] No specific cure has been discovered for homocystinuria; however, many people are treated using high doses of vitamin B6, which is a cofactor of CBS.
[18] Absence of Cystathionine beta synthase in mice provokes infertility due to the loss of uterine protein expression.