Spindle checkpoint
[15] Using its own observations, Zirkle[5] was the first to propose that "some (…) substance, necessary for the cell to proceed to anaphase, appears some minutes after C (moment of the arrival of the last chromosome to the metaphase plate), or after a drastic change in the cytoplasmic condition, just at C or immediately after C", suggesting that this function is located on kinetochores unattached to the mitotic spindle.
Just at the beginning of mitosis, both centrioles achieve their maximal length, recruit additional material and their capacity to nucleate microtubules increases.
Each chromatid has a special region, named the centromere, on top of which is assembled a proteic structure termed kinetochore, which is able to stabilize the microtubule plus end.
Conversion from lateral to end-on attachments allows the growth and shrinkage of the microtubule plus-ends to be converted into forces that push and pull chromosomes to achieve proper bi-orientation.
Merotelic orientation (characterized by the absence of tension between sister kinetochores) is frequent at the beginning of mitosis, but the protein Aurora B (a kinase conserved from yeast to vertebrates) detects and eliminates this type of anchoring.
Genetic and biochemical studies in yeast and in egg's extracts in Xenopus laevis identified a polyprotein complex as an essential player in sister chromatids cohesion (see the review from Hirano in 2000[29]).
In yeast, cohesin binds to preferential sites along chromosome arms, and is very abundant close to the centromeres, as it was shown in a study using chromatin immunoprecipitation.
[33][34] More recent studies indicate that the RNAi machinery regulates heterochromatin establishment, which in turn recruits cohesin to this region, both in S. pombe[35] and in vertebrate cells.
Centromeric cohesion resists the forces exerted by spindle microtubules towards the poles, which generate tension between sister kinetochores.
In turn, this tension stabilizes the attachment microtubule-kinetochore, through a mechanism implicating the protein Aurora B (a review about this issue : Hauf and Watanabe 2004[41]).
[47] Although this machinery is conserved through evolution,[48][49] in vertebrates most cohesin molecules are released in prophase, independently of the presence of the APC/C, in a process dependent on Polo-like 1 (PLK1) and Aurora B.
[56] In mouse oocytes, DNA damage induces meiotic prophase I arrest that is mediated by the spindle assembly checkpoint.
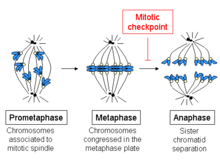
[58] The spindle assembly checkpoint (SAC) is an active signal produced by improperly attached kinetochores, which is conserved in all eukaryotes.
Merotelic attachments generate sufficient tension and are not detected by the SAC, and without correction, may result in chromosome mis-segregation due to slow chromatid migration speed.
Upon microtubule-kinetochore attachment, a mechanism of stripping via a dynein-dynein motor complex transports spindle checkpoint proteins away from the kinetochores.
In fact, aneuploidy is the most common characteristic of human solid tumors and thus the spindle assembly checkpoint might be regarded as a possible target for anti-tumour therapy.
[66] This is a much underappreciated fact since mutations in specific genes known as oncogenes or tumor suppressor are primarily thought to be behind genetic instability and tumorigenesis.
Usually the various checkpoints in the cell cycle take care of genomic integrity via highly conserved redundant mechanisms that are important for maintaining cellular homeostasis and preventing tumorigenesis.
In the hematological cancers such as multiple myeloma cytogenetic abnormalities are very common due to the inherent nature of DNA breaks needed for immunoglobulin gene rearrangement.
[73][74] However, recent studies indicate that what seems to happen is a more complicated scenario: aneuploidy would drive a high incidence of tumorigenesis only when alterations in the levels of specific mitotic checkpoint components (either reduction or overexpression) in tissues is also inducing other defects able to predispose them to tumors.
For some mitotic checkpoint components, it is known that they are implicated in functions outside mitosis: nuclear import (Mad1), transcriptional repression (Bub3), and cell death, DNA damage response, aging, and megakaryopoiesis for BubR1.
Survivin, a member of the inhibitor of apoptosis (IAP) family, is localized in pools at microtubules of the mitotic spindle near the centrosomes and at the kinetochores of metaphase chromosomes.
Not only does survivin inhibit apoptosis to promote tumorigenesis, but it has been implicated (through experimental knockout mice) as an important regulator of chromosome segregation, and late stage mitosis similar to its role in more primitive organisms.
[77] Other aspects of the spindle assembly checkpoint such as kinetochore attachment, microtubule function, and sister chromatid cohesion are likely to be defective as well to cause aneuploidy.
Cancer cells have been observed to divide in multiple directions by evading the spindle assembly checkpoint resulting in multipolar mitoses.
[78] The multipolar metaphase-anaphase transition occurs through an incomplete separase cycle that results in frequent nondisjunction events which amplify aneuploidy in cancer cells.
[81] The kinase gene Aurora A when amplified acts as an oncogene overriding the SAC leading to abnormal initiation of anaphase and subsequent aneuploidy and also resistance to TAXOL .
[82] Excitingly, a small molecule inhibitor of Aurora A has shown antitumor effects in an in vivo model suggesting that this might be a good target for further clinical development.
[81] Survivin is also an attractive molecular target for clinical therapeutic development as it acts as a major node in a multitude of pathways, one of which is spindle formation and checkpoint control.
These inhibitors, which have recently entered clinical trials, cause mitotic arrest and by engaging the spindle assembly checkpoint and induce apoptosis.