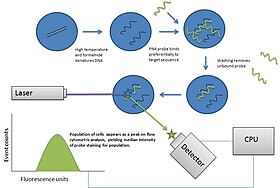
Flow cytometry
Flow cytometry (FC) is a technique used to detect and measure the physical and chemical characteristics of a population of cells or particles.
The first label-free high-frequency impedance flow cytometer based on a patented microfluidic "lab-on-chip", Ampha Z30, was introduced by Amphasys (2012).
Early flow cytometers were, in general, experimental devices, but technological advances have enabled widespread applications for use in a variety of both clinical and research purposes.
[12] Increasing the number of lasers and detectors allows for multiple antibody labeling, and can more precisely identify a target population by their phenotypic markers.
Spectral flow cytometry uses prisms or diffraction gratings to disperse the emitted light of a marker across a detector array.
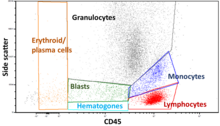
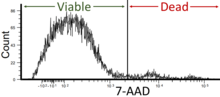
The regions on these plots can be sequentially separated, based on fluorescence intensity, by creating a series of subset extractions, termed "gates."
Because different fluorescent dyes' emission spectra overlap,[20][21] signals at the detectors have to be compensated electronically as well as computationally.
Once the data is collected, there is no need to stay connected to the flow cytometer and analysis is most often performed on a separate computer.
[citation needed] Recent progress on automated population identification using computational methods has offered an alternative to traditional gating strategies.
Representative automated methods include FLOCK[22] in Immunology Database and Analysis Portal (ImmPort),[23] SamSPECTRAL[24] and flowClust[25][26][27] in Bioconductor, and FLAME[28] in GenePattern.
T-Distributed Stochastic Neighbor Embedding (tSNE) is an algorithm designed to perform dimensionality reduction, to allow visualization of complex multi-dimensional data in a two-dimensional "map".
Fluorescence minus one (FMO) controls are important for data interpretation when building multi-color panels – in which a cell is stained with multiple fluorochromes simultaneously.
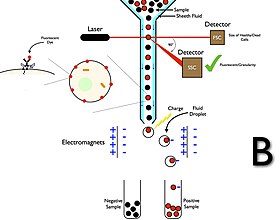
[13][15] The first prototype sorter was built at the Los Alamos National Laboratory (LANL) in 1965 by physicist Mack J. Fulwyler by joining a Coulter volume sensor with the newly invented ink jet printer.
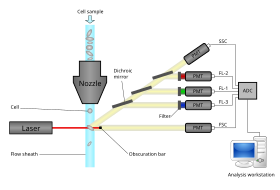
The collection process starts when a sample is injected into a stream of sheath fluid that passes through the flow cell and laser intercepts.
Immunophenotyping is the analysis of heterogeneous populations of cells using labeled antibodies[36] and other fluorophore containing reagents such as dyes and stains.
[citation needed] Mass cytometry overcomes the fluorescent labeling limit by utilizing lanthanide isotopes attached to antibodies.
Although this method permits the use of a large number of labels, it currently has lower throughput capacity than flow cytometry.
[39] In addition to the ability to label and identify individual cells via fluorescent antibodies, cellular products such as cytokines, proteins, and other factors may be measured as well.
The amount of the analyte captured is detected via a biotinylated antibody against a secondary epitope of the protein, followed by a streptavidin-R-phycoerythrin treatment.
The fluorescent intensity of R-phycoerythrin on the beads is quantified on a flow cytometer equipped with a 488 nm excitation source.
[40][41] This patented technology allows a highly accurate cell analysis and provides additional information like membrane capacitance and viability.
Measurable factors in cellular analysis and flow cytometry include a diverse set of traits and indicators that provide important insights into cell biology and function.
Flow cytometry techniques can quantify and evaluate these factors, allowing researchers to investigate and analyze various aspects of cells.
[45] It has broad application in medicine especially in transplantation, hematology, tumor immunology and chemotherapy, prenatal diagnosis, genetics and sperm sorting for sex preselection.
Flow cytometry is widely applied to detect sperm cells abnormality associated with DNA fragmentation[46] in male fertility assays.
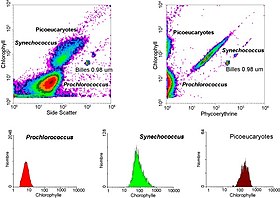
This research started in 1981 when Clarice Yentsch used flow cytometry to measure the fluorescence in a red tide producing dinoflagellate.
[55] The next year researchers published flow cytometric measurements of multiple algal species which could be distinguished based on their fluorescence characteristics.
[58] Flow cytometry also allowed marine researchers to distinguish between dimly-fluorescing Prochlorococcus and heterotrophic microorganisms, a distinction that is difficult with microscopy-based assessments.
One such assay to determine the cell proliferation is the tracking dye carboxyfluorescein diacetate succinimidyl ester (CFSE).
[68] In literature, this powerful technique of flow cytometry and CFSE has been used to find the efficiency of T-cells in killing the target cells in cancer such as leukemia.