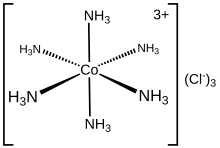
Coordinate covalent bond
An example of a dative covalent bond is provided by the interaction between a molecule of ammonia, a Lewis base with a lone pair of electrons on the nitrogen atom, and boron trifluoride, a Lewis acid by virtue of the boron atom having an incomplete octet of electrons.
In common usage, the prefix dipolar, dative or coordinate merely serves to indicate the origin of the electrons used in creating the bond.
The dative bond is also a convenience in terms of notation, as formal charges are avoided: we can write D: + []A ⇌ D → A rather than D+–A– (here : and [] represent the lone-pair and empty orbital on the electron-pair donor D and acceptor A, respectively).
The notation is sometimes used even when the Lewis acid-base reaction involved is only notional (e.g., the sulfoxide R2S → O is rarely if ever made by reacting the sulfide R2S with atomic oxygen O).
[7] The ammonia-borane adduct (H3N → BH3) is given as a classic example: the bond is weak, with a dissociation energy of 31 kcal/mol (cf.
153 pm for ethane), and the molecule possesses a dipole moment of 5.2 D that implies a transfer of only 0.2 e– from nitrogen to boron.
However, aside from clear-cut examples, there is considerable dispute as to when a particular compound qualifies and, thus, the overall prevalence of dative bonding (with respect to an author's preferred definition).
[8][9][10] Some non-obvious examples where dative bonding is claimed to be important include carbon suboxide (O≡C → C0 ← C≡O), tetraaminoallenes (described using dative bond language as "carbodicarbenes"; (R2N)2C → C0 ← C(NR2)2), the Ramirez carbodiphosphorane (Ph3P → C0 ← PPh3), and bis(triphenylphosphine)iminium cation (Ph3P → N+ ← PPh3), all of which exhibit considerably bent equilibrium geometries, though with a shallow barrier to bending.