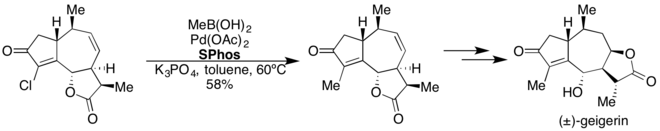
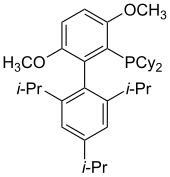
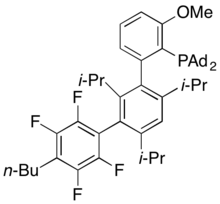
Dialkylbiaryl phosphine ligands
[1] In addition to these Pd-based processes, their use has also been extended to transformations catalyzed by nickel,[2] gold,[3][4][5] silver,[6] copper,[7] rhodium,[8][9] and ruthenium,[10][11] among other transition metals.
[12] Dialkylbiaryl phosphine ligands were first described by Stephen L. Buchwald in 1998 for applications in palladium-catalyzed coupling reactions to form carbon-nitrogen and carbon-carbon bonds.
[15][16] Their enhanced catalytic activity over other ligands in palladium-catalyzed coupling reactions have been attributed to their electron-richness, steric bulk, and some special structural features.
The lower ring of the biphenyl system, ortho to the phosphino group, is also a key structural feature.
Numerous crystallographic studies have indicated that it behaves as a hemilabile ligand and is believed to play a role in stabilizing the highly reactive, formally 12-electron L–Pd0 intermediate during the catalytic cycle.
Extensive experimentation by the Buchwald group has shown that further minor changes to the structure of these ligands can dramatically alter their catalytic activity in cross coupling reactions with different substrates.
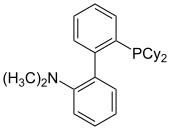
[18][19] DavePhos, the first reported dialkylbiaryl phosphine ligand, was initially used in Pd-catalyzed Suzuki-Miyaura cross-coupling reactions as well as Buchwald-Hartwig aminations.
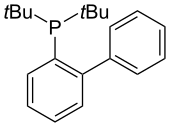
t-BuDavePhos has been shown to be an even more reactive variant of DavePhos in the room temperature Suzuki-Miyaura coupling of aryl bromides and chlorides.
This reaction has been conducted on a kilogram scale, and no specific palladium-removal treatment was required as the excess imidazole present in the final amide coupling step coordinated to the Pd and generated a removable byproduct.
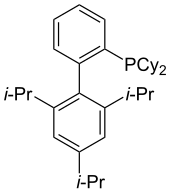
[49] The bulky AdBrettPhos can be used in the amidation of five-membered heterocyclic halides that contain multiple heteroatoms (such as haloimidazoles and halopyrazoles).
[53] It has also been used to synthesize palladacycle precatalysts for Negishi coupling of secondary alkylzinc reagents with aryl halides.
The resulting L–PdII(Ar)X OAC is electrophilic such that it reacts with a nucleophile and forms C–C and C–heteroatom bonds, after reductive elimination.