Negishi coupling
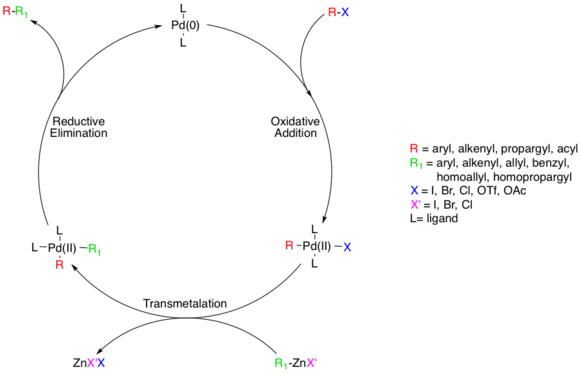
The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process.
The Negishi coupling finds common use in the field of total synthesis as a method for selectively forming C-C bonds between complex synthetic intermediates.
[7] Alongside Richard F. Heck and Akira Suzuki, El-ichi Negishi was a co-recipient of the Nobel Prize in Chemistry in 2010, for his work on "palladium-catalyzed cross couplings in organic synthesis".
[8] This step proceeds with aryl, vinyl, alkynyl, and acyl halides, acetates, or triflates, with substrates following standard oxidative addition relative rates (I>OTf>Br≫Cl).
[8] Transmetalation is usually rate limiting and a complete mechanistic understanding of this step has not yet been reached though several studies have shed light on this process.
In one Negishi model system the formation of homocoupling was found to be the result of a second transmetalation reaction between the diarylmetal intermediate and arylmetal halide:[16]
Kochi and Morrell provided evidence for this by isolating NiII complex Ni(PEt3)2(Me)(o-tolyl), which did not undergo reductive elimination quickly enough to be involved in this elementary step.
[27] Reactions between sp3-sp3 centers are often more difficult; however, adding an unsaturated ligand with an electron withdrawing group as a cocatalyst improved the yield in some systems.
[34][35] In 2003 Novartis employed a Negishi coupling in the manufacture of PDE472, a phosphodiesterase type 4D inhibitor, which was being investigated as a drug lead for the treatment of asthma.
[36] The Negishi coupling was used as an alternative to the Suzuki reaction providing improved yields, 73% on a 4.5 kg scale, of the desired benzodioxazole synthetic intermediate.
[37] Where the Negishi coupling is rarely used in industrial chemistry, a result of the aforementioned water and oxygen sensitivity, it finds wide use in the field of natural products total synthesis.
The increased reactivity relative to other cross-coupling reactions makes the Negishi coupling ideal for joining complex intermediates in the synthesis of natural products.
[38] The major drawback of the Negishi coupling, aside from its water and oxygen sensitivity, is its relative lack of functional group tolerance when compared to other cross-coupling reactions.
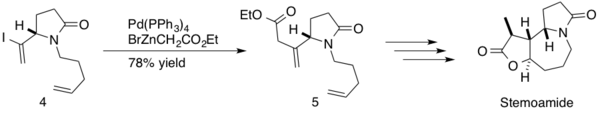
[40] The reaction was implemented mid-synthesis, forming an sp3-sp2 c-c bond between β,γ-unsaturated ester and an intermediate diene 4 with a 78% yield of product 5.
These toxic alkaloids display modulatory effects on voltage-dependent sodium channels, resulting in cardiotonic and myotonic activity.
Inhibition of pol B in conjunction with other chemotherapy drugs may increase the cytotoxicity of these chemotherapeutics, leading to lower effective dosages.
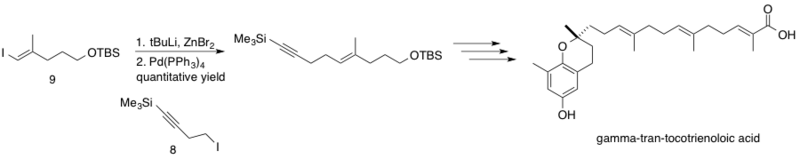
[43] The reaction proceeded with quantitative yield, coupling fragments mid-synthesis en route to the stereoselectively synthesized natural product δ-trans-tocotrienoloic acid.
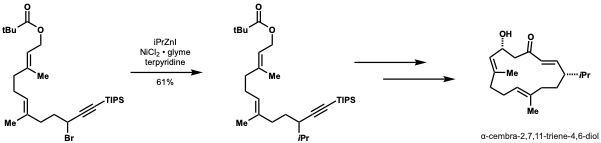
This method would be highly adaptable in this application for diversification and installing other alkyl groups to enable structure-activbity relationship (SAR) studies.
[44]Kirschning and Schmidt applied nickel catalyzed negishi cross-coupling to the first total synthesis of carolactone.