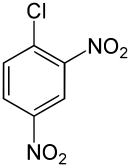
2,4-Dinitrochlorobenzene
[2] DNCB is produced commercially by the nitration of p-nitrochlorobenzene with a mixture of nitric and sulfuric acids.
Other methods afford the compound less efficiently include the chlorination of 1,3-dinitrobenzene, nitration of o-nitrochlorobenzene and the dinitration of chlorobenzene.
[3] By virtue of the two nitro substituents, the chloride in DNCB is particularly susceptible to nucleophilic substitution, at least relative to simple chlorobenzene.
[4][5][6] In one example, DNCB is as a substrate in Glutathione S-Transferase, relevant to activity assays.
[7] DNCB induces a type IV hypersensitivity reaction in almost all people exposed to it, so it is used medically to assess the T cell activity in patients.