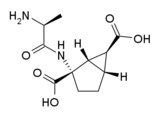
Eglumetad
[20] Eglumetad also interferes in the hypothalamic–pituitary–adrenal axis, with chronic oral administration of this drug leading to markedly reduced baseline cortisol levels in bonnet macaques (Macaca radiata); acute infusion of eglumetad resulted in a marked diminution of yohimbine-induced stress response in those animals.
[21] In human adrenocortical cells, eglumetad has been shown to down-regulate intracellular cyclic AMP (cAMP) and steroidogenesis, with a significant decrease in aldosterone and cortisol production.
[22] Development of this drug and related compounds is continuing, with several clinical trials completed and more planned.
Poor oral bioavailability of the original formulation led to limited efficacy in the initial human trials,[23] and so the prodrug form LY544344 (talaglumetad) did seem to be a more likely drug candidate for further development.
[24][25][26][27] However a clinical trial of LY544344 was discontinued early based on findings of convulsions in preclinical studies.