Entropy (classical thermodynamics)
The term was introduced by Rudolf Clausius in the mid-19th century to explain the relationship of the internal energy that is available or unavailable for transformations in form of heat and work.
Entropy predicts that certain processes are irreversible or impossible, despite not violating the conservation of energy.
He showed that the thermodynamic entropy is k ln Ω, where the factor k has since been known as the Boltzmann constant.
Differences in pressure, density, and temperature of a thermodynamic system tend to equalize over time.
In an isolated system, such as the room and ice water taken together, the dispersal of energy from warmer to cooler regions always results in a net increase in entropy.
One of them is mixing of two or more different substances, occasioned by bringing them together by removing a wall that separates them, keeping the temperature and pressure constant.
In the important case of mixing of ideal gases, the combined system does not change its internal energy by work or heat transfer; the entropy increase is then entirely due to the spreading of the different substances into their new common volume.
According to the Clausius equality, for a closed homogeneous system, in which only reversible processes take place, With
, called entropy, may be defined which satisfies The thermodynamic state of a uniform closed system is determined by its temperature T and pressure P. A change in entropy can be written as The first contribution depends on the heat capacity at constant pressure CP through This is the result of the definition of the heat capacity by δQ = CP dT and T dS = δQ.
For example, for pure substances, one can take the entropy of the solid at the melting point at 1 bar equal to zero.
From a more fundamental point of view, the third law of thermodynamics suggests that there is a preference to take S = 0 at T = 0 (absolute zero) for perfectly ordered materials such as crystals.
Entropy values of important substances may be obtained from reference works or with commercial software in tabular form or as diagrams.
For example, Fig.2 shows the TS-diagram of nitrogen,[3] depicting the melting curve and saturated liquid and vapor values with isobars and isenthalps.
For example, consider an insulating rigid box divided by a movable partition into two volumes, each filled with gas.
An irreversible process degrades the performance of a thermodynamic system, designed to do work or produce cooling, and results in entropy production.
Clausius' identification of S as a significant quantity was motivated by the study of reversible and irreversible thermodynamic transformations.
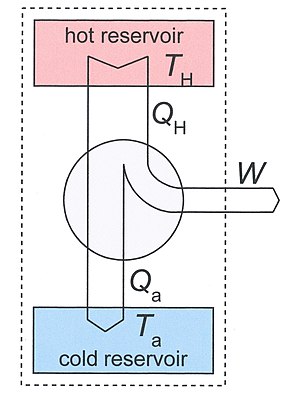
A heat engine is a thermodynamic system that can undergo a sequence of transformations which ultimately return it to its original state.
Finally This equation tells us that the production of work is reduced by the generation of entropy.