Ethyl pyruvate
This study concluded three things: First, ethyl pyruvate prevents the severe acute pancreatitis-induced hepatic expression of tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β).
This experiment concluded that the pyruvate treatments proved beneficial neurologically post-cortical contusion injury (CCI).
Ethyl pyruvate is a good antioxidant due to its α-ketocarboxylate structure, which allows it to reduce hydrogen peroxide to water and scavenge the hydroxyl radical through decarboxylation.
[10] Overall, ethyl pyruvate has been found to be beneficial in wound healing, liver disease, pancreatitis, and spinal cord repair.
Relating to health, there are still many researchers using ethyl pyruvate in their projects pertaining to myocardial ischemia, reperfusion, and human gastric cancer.
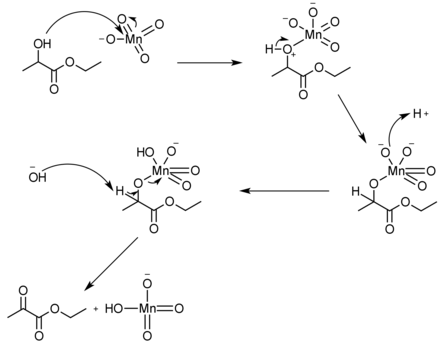
There are many different reagents that can be used to push the reaction forward to yield in excess of 98%, such as using potassium permanganate and aluminum sulfate hydrate in dichloromethane solvent.
Enantioselective reactions are extremely important in chemistry, as the formation of optically pure products is especially useful in the food, pharmaceutical, and agrochemical industries.