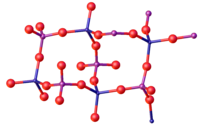
Iron(III) phosphate
At high pressures, a phase change occurs to a more dense structure with octahedral Fe centres.
In the two polymorphs of the dihydrate, the Fe centre is octahedral with two mutually cis water ligands.
Its presence is partially responsible for the corrosion resistance of the iron pillar of Delhi.
It can also be used for bonding fabrics, wood, and other materials to iron or steel surfaces.
Iron(III) phosphate is not allowed as food additive in the European Union.