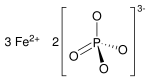
Iron(II) phosphate
Iron(II) phosphate, also ferrous phosphate,[3] Fe3(PO4)2, is an iron salt of phosphoric acid.
The mineral vivianite is a naturally occurring form of hydrated iron(II) phosphate.
It can be formed by the reaction of ferrous hydroxide with phosphoric acid to produce hydrated iron(II) phosphate.
Media related to Iron(II) phosphate at Wikimedia Commons
This inorganic compound–related article is a stub.